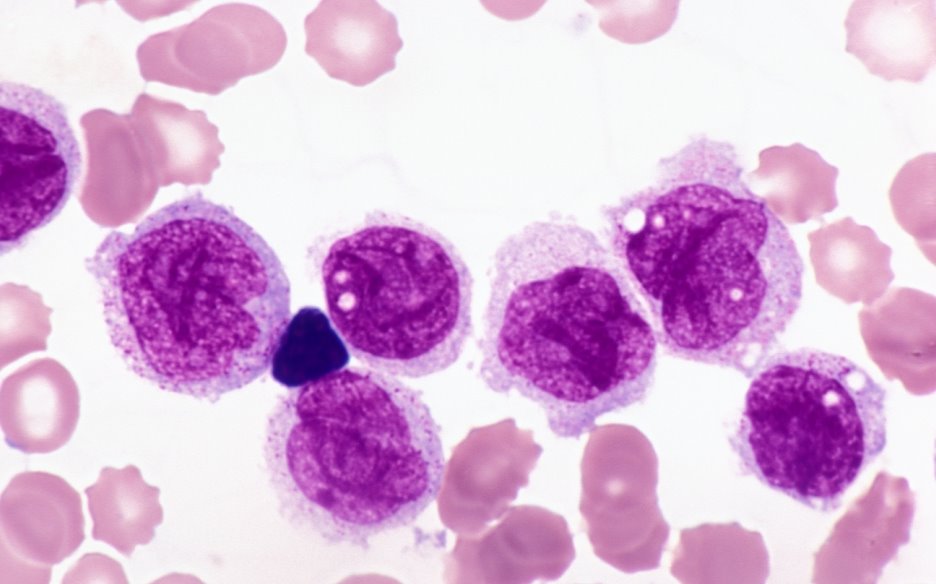
 Mutant hematopoietic stem cells in the bone marrow proliferate and fail to differentiate into white blood cells in Acute myeloid leukemia (AML). FLT3 is one of the most common genes mutated; however mutated FLT3 alone is not sufficient in leading to AML. Researchers have found that some patients with mutated FLT3 often have elevated levels of another protein RUNX1, which has been believed to be a tumor suppressor. Surprisingly, they reported that coexpression of RUNX1 with FLT3 blocks the differentiation of white blood cells by inducing another transcription factor Hhex. They proposed RUNX1 may suppress activation of AML initially; however, FLT3 inactivation may lead to RUNX1 activation that would promote tumor growth. Reducing RUNX1 in cancer stem cells may be deleterious but not in normal cells thus providing a selective target for potential interventions.
Mutant hematopoietic stem cells in the bone marrow proliferate and fail to differentiate into white blood cells in Acute myeloid leukemia (AML). FLT3 is one of the most common genes mutated; however mutated FLT3 alone is not sufficient in leading to AML. Researchers have found that some patients with mutated FLT3 often have elevated levels of another protein RUNX1, which has been believed to be a tumor suppressor. Surprisingly, they reported that coexpression of RUNX1 with FLT3 blocks the differentiation of white blood cells by inducing another transcription factor Hhex. They proposed RUNX1 may suppress activation of AML initially; however, FLT3 inactivation may lead to RUNX1 activation that would promote tumor growth. Reducing RUNX1 in cancer stem cells may be deleterious but not in normal cells thus providing a selective target for potential interventions.
References:
1.https://www.sciencedaily.com/releases/2017/02/170217095930.htm
2.Kira Behrens, Katrin Maul, Nilgün Tekin, Neele Kriebitzsch, Daniela Indenbirken, Vladimir Prassolov, Ursula Müller, Hubert Serve, Jörg Cammenga, Carol Stocking. RUNX1 cooperates with FLT3-ITD to induce leukemia. The Journal of Experimental Medicine, 2017; jem.20160927 DOI
Article Summary Courtesy: Jennifer Lu and Waleed Khan

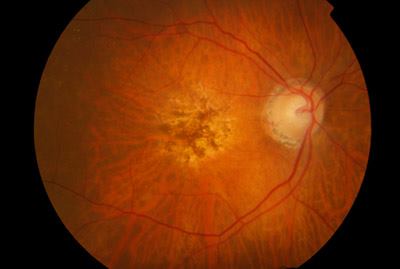
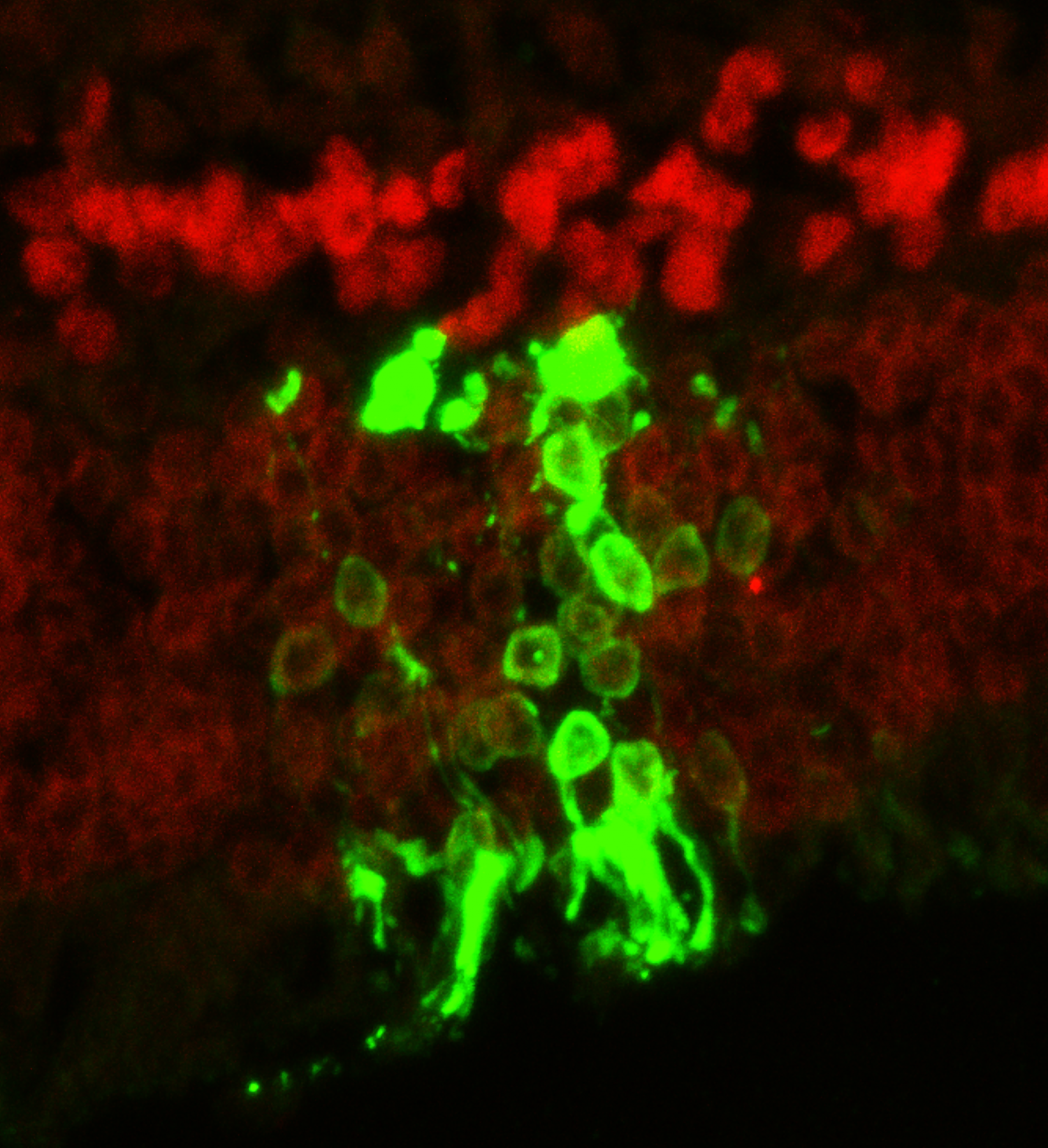 One of the major obstacles in stem cell transplantation is preventing immune rejection that would recognize transplanted cells as foreign and attack them leading to disappointing long-term outcomes. Although the eye and the brain have been considered as exceptions from immune surveillance, Zhu et al. showed that immune rejection was one of the major contributors to degenerated transplanted photoreceptors in blind mice after temporarily restoring sights. Transplantation of photoreceptors derived from stem cells into a strain of immunodeficient IL2 receptor gamma null mice was found to have a 10-fold increase in living transplanted cells that differentiated into mature photoreceptors expressing opsins. Even after nine months to one year after transplantation, the eyes of the mice were shown to be functional and transmitting signals to the brain. Their results showed that to improve longevity of transplanted retinal cells in restoring eye sight, immunosuppression is crucial and specific inhibitors against the IL2 gamma receptor could be developed to selectively suppress immune rejection to retinal cells instead of systemic immunosuppression.
One of the major obstacles in stem cell transplantation is preventing immune rejection that would recognize transplanted cells as foreign and attack them leading to disappointing long-term outcomes. Although the eye and the brain have been considered as exceptions from immune surveillance, Zhu et al. showed that immune rejection was one of the major contributors to degenerated transplanted photoreceptors in blind mice after temporarily restoring sights. Transplantation of photoreceptors derived from stem cells into a strain of immunodeficient IL2 receptor gamma null mice was found to have a 10-fold increase in living transplanted cells that differentiated into mature photoreceptors expressing opsins. Even after nine months to one year after transplantation, the eyes of the mice were shown to be functional and transmitting signals to the brain. Their results showed that to improve longevity of transplanted retinal cells in restoring eye sight, immunosuppression is crucial and specific inhibitors against the IL2 gamma receptor could be developed to selectively suppress immune rejection to retinal cells instead of systemic immunosuppression. More than 20 million Canadians suffer from digestive disorders every year
More than 20 million Canadians suffer from digestive disorders every year