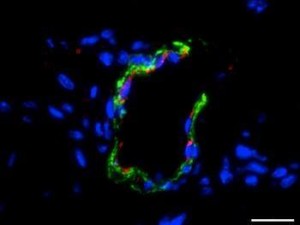
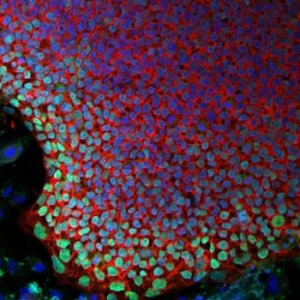
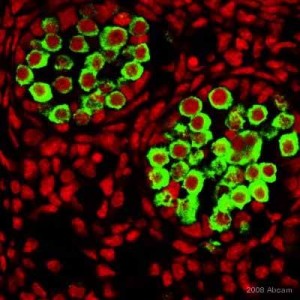
A team led by Jeffrey G. Jacot, associate professor of bioengineering at Rice University recently demonstrated that amniotic stem cells have the potential to be used as a therapeutic for heart diseases. Amniotic stem cells are cells that are taken from the amniotic fluid and have the ability to give rise to a wide variety of cells, including endothelial cells that can form blood vessels.
The team used hydrogels seeded with amniotic stem cells to see if it can promote the formation of new blood vessels. Hydrogel 3D scaffolds are widely used in medicine to deliver drugs and one form of it that contains fibrin can promote angiogenesis. The team modified the hydrogel with polyethylene glycol that makes it firm and long lasting.
The team then injected mice with either the hydrogel or with the hydrogel seeded with the stem cells. Growth factors were also added to promote cellular differentiation. The team found that the hydrogel seeded with the stem cells led to greater growth of blood vessels. This is significant since these fibrin hydrogels in combination with amniotic stem cells can be used to treat heart diseases. At the same time, if the stem cells are taken from the amniotic fluid of the infant, which has to receive treatment, it will reduce the chances of immune rejection. Right now, the team is working on developing biocompatible patches that can be used to treat infants with defects in their heart.
Article summary courtesy by Waleed Khan
Article: Medical News Today
Reference:
In situ vascularization of injectable fibrin/poly(ethylene glycol) hydrogels by human amniotic fluid-derived stem cells, Omar M. Benavides et al., Journal of Biomedical Materials Research Part A, doi:10.1002/jbm.a.35402, published online 9 February 2015